Antibody Drug Conjugate (ADC) Targets
Antibody Drug Conjugates (ADCs) were one of the most popular drug classes presented at the ASCO annual meeting earlier this month.
NEW YORK, NEW YORK, UNITED STATES, June 27, 2023/ EINPresswire.com/ -- One of the largest classes of emerging cancer medicines presented as the American Society of Clinical Oncology (ASCO) annual meeting this year were Antibody Drug Conjugates (ADCs). OBiS, a private consulting firm based in New York City and Oloron-Sainte-Marie France has developed an analytics spreadsheet tool which profiles key drugs showcased at ASCO by mechanism of action and target. According to OBiS, ASCO23 abstracts covered 38 ADCs of which: (A) Nine ADCs are currently approved; (B) Seven ADCs are in late phase clinical development; (C) 19 ADCs are in early phase clinical trials, mostly First-in-Human studies; and (D) three are preclinical ADCs.
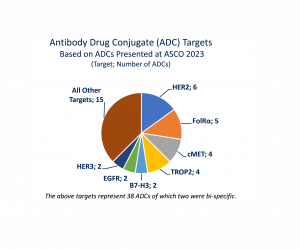
The ADCs presented at ASCO23 involve 22 different drug targets with the most popular being: HER2 (6 ADCs); FolRα (5 ADCs); cMET (4 ADCs); TROP2 (4 ADCs); B7-H3 (2 ADCs); EGFR (2 ADCs); HER3 (2 ADCs). This level of target diversity offers the opportunity to address a wide range of both hematological, sarcoma and solid tumor cancers such as bladder, breast, colorectal, gynecological, and lung.
ADCs consist of three key components: A targeting antibody, usually based on tumor associated antigens (TAAs); a cytotoxic payload (also referred to as a warhead); and a linker which controls the release of the cytotoxic payload upon binding of the ADC antibody with the tumor antigen. There are three main types of payloads: (1) microtubule-targeting (e.g. DM4, MMAE, MMAF) representing over half of the ASCO23 ADCs, (2) topoisomerase I (topo I) based representing about a third of the ASCO23 ADCs; and (3) DNA x-linkers (e.g. calicheamicin, tesirine). There was one ADC presented at ASCO23, mirzotamab clezutoclax (ABBV-155), currently being developed by AbbVie that uses a BCL-XL inhibitor as its payload to facilitate tumor apoptosis.
Many of the ADC payloads have been around for decades, but were previously found to be far too toxic for direct administration. For example the ADC Trodelvy® (sacituzumab govitecan) uses a SN-38 topo I payload. SN-38 is considered to be as much as 1,000 times more potent than the nearly three-decade old cancer drug, irinotecan (Camptosar®) which is a SN-38 prodrug: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6748572. As some prominent scientists have suggested, ADCs could potentially replace traditional chemotherapy leading to much better outcomes and quality of life for patients (Dr Lee Schwartzberg, Antibody Drug Conjugates: The end of chemotherapy | 2023 West Oncology Conference, March 13, 2023. https://www.youtube.com/watch?v=XLH4sxR9j-A).
Below is a summary of the ADCs presented at ASCO 2023.
(A) NINE APPROVED ADCs (displayed below by Target: Drug)
BCMA: Blenrep® (belantamab mafodotin) | CD19: Zynlonta® (loncastuximab tesirine) | CD22: Besponsa® (inotuzumab ozogamicin) | CD30: Adcetris® (brentuximab vedotin) | FolRα: Elahere® (mirvetuximab soravtansine) | HER2: Enhertu® (trastuzumab deruxtecan) and Kadcyla® (trastuzumab emtansine) | Nectin4: Padcev® (enfortumab vedotin) | TROP2: Trodelvy® (sacituzumab govitecan).
A total of 53 ASCO23 abstracts were reviewed by OBiS pertaining to the above approved ADCs of which two were the subject of ASCO Daily News publications. The first Daily News on these ADCs covered the SWOG-S1826 clinical trial (ASCO23 abstract #LBA4, NCT03907488) which compared the ADC Adcetris® (brentuximab vedotin) to the PD-1 checkpoint inhibitor Opdivo® (nivolumab) along with standard chemotherapy (doxorubicin, vinblastine, and dacarbazine) used with both agents. The study concluded that the nivolumab arm was better tolerated and thus has set the stage for nivolumab/AVD chemo to become the new standard of care for both adult and pediatric patients with newly diagnosed stage III through IV classic Hodgkin lymphoma. The abstract was presented in the Plenary Session by Dr Alex Francisco Herrera MD ( https://dailynews.ascopubs.org/do/swog-s1826-paves-way-nivolumab-plus-avd-chemotherapy-new-standard-care-advanced-stage).
The second ASCO Daily News publication covered the DESTINY-PanTumor-02 clinical trial (ASCO23 abstract #LBA3000, NCT04482309) which assessed the ADC Enhertu® (trastuzumab deruxtecan, a.k.a. T-Dxd) in six different solid tumors (urothelial bladder, biliary tract, cervical, endometrial, ovarian, and pancreatic) and a rare cohort of head and neck cancers and intestinal adenocarcinoma. While the study is still in progress, interim results are showing efficacy in a broad range of tumors with the exception of pancreatic cancer which had the lowest efficacy resulting in the early stop of this cohort. The abstract was presented in the Oral Abstract Session by Dr Funda Meric-Bernstam MD ( https://dailynews.ascopubs.org/do/destiny-pantumor-02-trastuzumab-deruxtecan-has-activity-against-range-her2-expressing).
(B) SEVEN PHASE II/III ADCs (displayed below by Target: Drug)
cMET: telisotuzumab vedotin (ABBV-399) | HER2: disitamab vedotin (RC48-ADC) | HER3: patritumab deruxtecan (Dato-DXd, U3-1402) | NaPi2b: upifitamab rilsodotin (XMT-1536) | ROR1: zilovertamab vedotin (MK-2140) | ROR2: ozuriftamab vedotin (BA3021) | TROP2: datopotamab deruxtecan (DS-1062).
There were two Oral Abstract Sessions covering the above late phase non-approved ADCs. The first was based on the phase II trial in HER2(-)/HER3(+) metastatic breast cancer patients (ASCO23 abstract #1004, NCT04699630) which involved monotherapy with the ADC patritumab deruxtecan (Dato-DXd, U3-1402). Dato-DXd was granted breakthrough therapy designation by the US FDA in December 2021 and specifically targets HER3. Data from Part A (60 heavily pre-treated ER+ and TNBC patients) was presented by Dr Erika P. Hamilton of the Sarah Cannon Research Institute indicating significant clinical activity in a broad range of HER3 expression levels ( https://meetings.asco.org/abstracts-presentations/219699).
The second Oral Abstract Session on the late phase ADCs covered the TROPION-Lung02 phase 1b clinical trial in NSCLC (ASCO23 abstract #9004, NCT04526691) which involved the ADC datopotamab deruxtecan (DS-1062) along with the PD-1 checkpoint inhibitor, Keytruda® (pembrolizumab) and standard carbo/cis chemotherapy. The study was presented by Dr Yasushi Goto of the National Cancer Center Hospital, Tokyo, Japan, and according to the abstract has demonstrated “tolerable safety” and “notable” activity in the first-line setting ( https://meetings.asco.org/abstracts-presentations/219194).
(C) 19 EARLY PHASE ADCs (displayed below by Target: Drug)
AXL: mecbotamab vedotin (BA3011) | B7-H3: HS-20093 and mirzotamab clezutoclax (ABBV-155) | B7-H4: XMT-1660 | CEACAM5: tusamitamab ravtansine | CLDN18.2: SYSA1801 | cMET x EGFR (bispecific): AZD9592 | cMET: ABBV-400 and MYTX-011 | EGFR x HER3 (bispecific): BL-B01D1 | FolRα: luveltamab tazevibulin (STRO-002) and PRO1184 | HER2: BB-1701, DB-1303 and FS-1502 | ITGB6: SGN B6A | SEZ6: ABBV-011 | TROP2: ESG401 and SKB264.
One abstract for each of the 19 early phase ADCs was reviewed by OBiS of which three were presented during the ASCO23 Oral Abstract Session. The oral abstract for the SEZ6 targeting ADC, ABBV-011, was also the subject of an ASCO Daily News publication ( https://dailynews.ascopubs.org/do/adc-novel-sez6-target-well-tolerated-preliminary-efficacy-small-cell-lung-cancer-phase) and presented by Dr. Daniel Morgensztern of the Washington University School of Medicine (ASCO23 abstract # 3002, NCT03639194). As Dr Morgensztern explained, SEZ6 is an “appealing” target as it is highly expressed in neuroendocrine tumors, including SCLC and certain neuronal tissue, but generally has a low-level expression in normal tissues.
The other two Oral Abstract Presentations covered (1) Luveltamab tazevibulin (STRO-002), an anti-folate receptor alpha (FolRα) ADC being studied in recurrent epithelial ovarian cancer (ASCO23 abstract #5508, NCT03748186) presented by Dr. Ana Oaknin MD, PhD ( https://meetings.asco.org/abstracts-presentations/220286) and (2) The EGFRxHER3 bispecific ADC, BL-B01D1, in advanced solid tumors (ASCO23 abstract #3001, NCT05194982) presented by Dr. Li Zhang MD ( https://meetings.asco.org/abstracts-presentations/218492). In both cases, these studies have demonstrated encouraging efficacy and safety profiles to further advance these ADCs clinically.
(D) THREE PRECLINICAL ADCs
There were three preclinical ADCs included in the ASCO23 annual meeting as Publications Only. Two of which target FolRα: farletuzumab ecteribulin (MORAb-202) and MORAb-109 being studies pre-clinically in rare gynecologic cancers (ASCO23 abstract #e17634, https://meetings.asco.org/abstracts-presentations/223505). The other pre-clinical ADC presented, RGX-019, targets MERTK (ASCO23 abstract #e15103, https://meetings.asco.org/abstracts-presentations/225202) and is being evaluated in both liquid and solid cancers. A targeted MERTK ADC approach is hopefully able to avoid adverse effects associated with retinal degeneration that have been noted in earlier MERTK targeted therapies.
OTHER CONJUGATE BASED THERAPIES
There is a growing class of similar conjugate therapies involving alternatives to antibodies, such as peptides. Several examples presented at ASCO2023 include Peptide Drug Conjugates, such as CBX-12, sudocetaxel zendusortide (TH1902), and BDC-1001 a HER2-targeted immune-stimulating antibody conjugate (ISAC). Other non-antibody conjugates include: ELU001, a C'Dot Drug Conjugate, and WTX212, an Erythro-Drug Conjugate. Conjugate based radiotherapies are also starting to emerge, such as PNT2002, a radioligand PSMA targeted antibody conjugate for prostate cancer, recently granted US FDA fast track designation in April 2023.
ABOUT OBiS
OBiS is a privately owned New York City based healthcare analytics corporation offering a variety of products and services in business development, forecasting, new product planning, key opinion leader identification, market research, clinical trial, recruitment analytics and custom data services. OBiS serves primarily biotechnology and large pharmaceutical companies. OBiS was founded in 2002 by Rick Beasley, former Director of Oncology Market Research for Bristol-Myers Squibb. In 2020, OBiS opened a subsidiary office in Oloron-Sainte-Marie, France as a simplified joint-stock company (SAS) entity.
As part of its mission to explore new opportunities in drug development, OBiS has launched several exploratory projects covering personalized medicine (pharmacogenomics), French-Africa clinical development and French biotechnology facilitated growth programs. Additionally, a new program, termed the Artisan Brain Initiative is planned later this year to address the growing opportunities as well as concerns of advances in Artificial Intelligence (The Brain Initiative® is a registered trademark of the US National Institute of Health).
DISCLAIMER
The information contained in this press release is for educational purposes only and is not intended to provide any medical or financial investment advice. OBiS, Inc has no association or affiliation with ASCO other than as a paid non-member industry attendee at the ASCO 2023 annual meeting.
Richard Beasley
OBIS
+1 347-983-1975
email us here
Visit us on social media:
Twitter
LinkedIn